Introduction
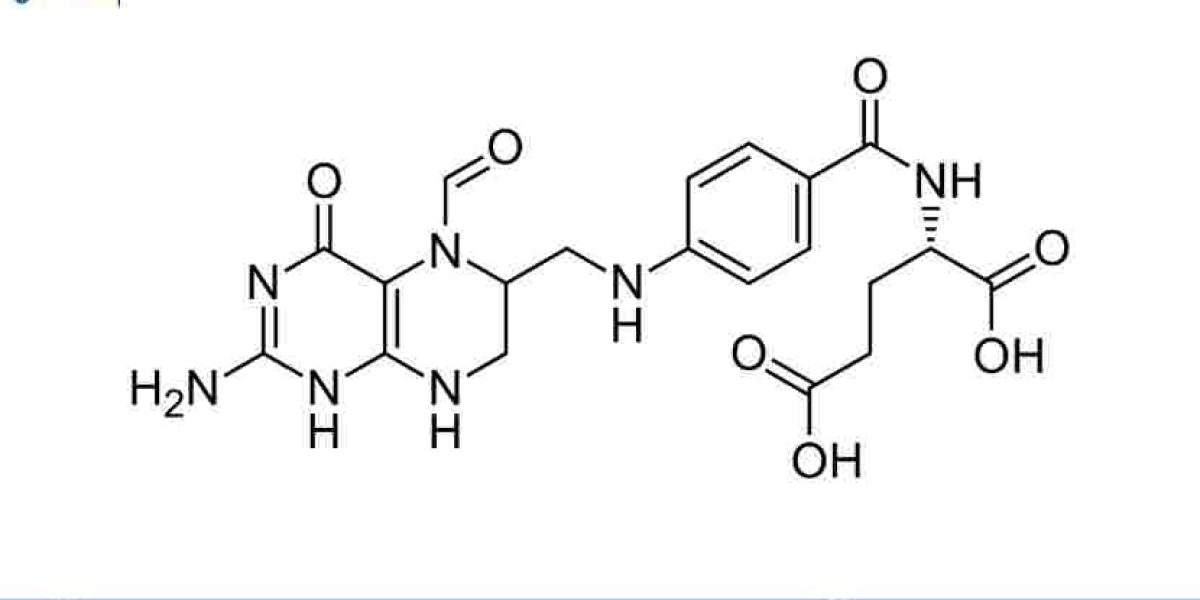
The Methotrexate Manufacturing Plant Project Report outlines the establishment of a production facility for methotrexate, an important pharmaceutical compound used in the treatment of various cancers, autoimmune diseases, and inflammatory conditions. Methotrexate, a type of antimetabolite, works by interfering with the growth of cancer cells, as well as inhibiting the immune system in conditions such as rheumatoid arthritis and psoriasis. The increasing global prevalence of cancer and autoimmune disorders, combined with the growing need for affordable treatments, makes the production of methotrexate a highly valuable and sustainable business venture.
This project report offers a detailed look at the necessary steps for setting up a Methotrexate Manufacturing Plant, including the market potential, production process, infrastructure requirements, and regulatory compliance. The goal is to provide key insights into the manufacturing process and how to ensure the plant’s profitability and sustainability in a highly regulated and competitive industry.
Executive Summary
The Methotrexate Manufacturing Plant will produce high-quality methotrexate tablets, injectable solutions, and other pharmaceutical forms. The facility will focus on producing the drug under stringent regulatory guidelines, ensuring that the final product meets international quality standards. The demand for methotrexate has been steadily increasing due to its wide range of medical applications, particularly in oncology and autoimmune therapies.
Key features of the plant include:
- Diverse Product Forms: The plant will produce methotrexate in various forms, including tablets, oral solutions, and injectables, to cater to a broad spectrum of patient needs.
- Adherence to GMP Standards: The plant will comply with Good Manufacturing Practices (GMP) to ensure product safety, efficacy, and consistency.
- Automation and Efficiency: Automated systems will be utilized in manufacturing, quality control, and packaging processes to ensure high production efficiency and reduce human error.
- Cost-Effective Production: The plant will focus on cost-efficient production methods to keep the final product affordable while maintaining high quality.
- International Market Potential: The plant will target both domestic and international markets, providing methotrexate to hospitals, clinics, and pharmaceutical distributors globally.
The project aims to meet the growing demand for methotrexate while ensuring high standards of quality, compliance, and profitability.
Get a Free Sample Report with Table of Contents@
Market Research and Feasibility Study
Market Demand
- Cancer Treatment: Methotrexate is widely used in the treatment of several cancers, including leukemia, breast cancer, and lymphoma. With the global cancer incidence steadily rising, the demand for methotrexate is expected to grow. Methotrexate remains one of the most widely prescribed chemotherapy agents due to its efficacy and relatively low cost compared to other cancer treatments.
- Autoimmune and Inflammatory Diseases: In addition to its use in oncology, methotrexate is a cornerstone drug for treating autoimmune diseases like rheumatoid arthritis, psoriasis, and Crohn’s disease. With the increasing prevalence of autoimmune diseases worldwide, particularly in aging populations, the demand for methotrexate as a disease-modifying anti-rheumatic drug (DMARD) is significant.
- Global Pharmaceutical Market: The methotrexate market is expected to expand globally due to its essential role in both cancer and autoimmune therapies. Developing regions, in particular, offer substantial growth potential as healthcare access improves and the demand for affordable medications rises.
Regulatory Environment
- FDA Approval: Methotrexate manufacturing is strictly regulated by agencies such as the U.S. Food and Drug Administration (FDA) in the United States, and similar regulatory bodies in other regions (e.g., EMA in Europe). Obtaining approval for manufacturing methotrexate requires compliance with strict quality control standards, including the production process, raw materials, packaging, and labeling.
- Good Manufacturing Practices (GMP): Compliance with GMP guidelines is essential in ensuring the safety, efficacy, and quality of methotrexate products. These standards govern everything from raw material sourcing to the final product distribution. The plant will need to undergo regular inspections by regulatory bodies to ensure it remains in compliance with GMP.
- Environmental Regulations: The manufacturing plant must adhere to environmental laws and standards to minimize waste, control emissions, and ensure proper disposal of chemicals used in production. Implementing sustainable manufacturing practices will not only ensure compliance but will also enhance the company’s reputation in the market.
Financial Feasibility
- Capital Investment: Setting up a methotrexate manufacturing plant requires significant capital investment, particularly for high-quality machinery, regulatory approvals, raw materials, and facility construction. Initial costs also include obtaining licenses, establishing laboratory facilities, and meeting GMP requirements.
- Operating Costs: Key operating expenses will include the procurement of raw materials (e.g., methotrexate salts, solvents), labor, utilities (such as electricity and water), packaging materials, and marketing. It is also important to allocate funds for R&D and continuous quality control.
- Revenue Streams: The primary revenue will come from the sale of methotrexate to healthcare providers, hospitals, and pharmaceutical distributors. Additionally, the plant could explore export opportunities, particularly in developing countries where access to affordable medications is growing in demand.
- Profit Margins: The pharmaceutical industry, particularly the generic drug sector, generally operates on lower margins, but high-volume sales and efficient production processes can lead to healthy profitability. As methotrexate is used globally, there is significant revenue potential in both established and emerging markets.
Manufacturing Process of Methotrexate
The production of methotrexate involves several critical steps, including the synthesis of raw materials, chemical reactions, formulation, and packaging. Below is a breakdown of the key stages in methotrexate production:
1. Raw Material Procurement
The primary raw materials required for methotrexate production include:
- Methotrexate salts: The active pharmaceutical ingredient (API) that forms the core of the drug.
- Solvents: Used in chemical reactions to facilitate synthesis.
- Excipients: Non-active ingredients used in formulation and for stability, including binders, preservatives, and fillers.
- Packaging materials: Containers and labeling for the final product.
2. Synthesis of Methotrexate
Methotrexate is synthesized through a multi-step chemical process, starting with precursor chemicals. The core chemical reaction involves the synthesis of methotrexate from various chemical compounds in a controlled environment. This step requires precise temperature, pressure, and chemical conditions to ensure the correct structure and purity of the final compound.
3. Formulation
Once the methotrexate is synthesized, it is formulated into its final dosage forms, such as tablets, oral solutions, or injectable forms. In this stage, the active ingredient is combined with excipients to create the right consistency, stability, and bioavailability. The formulation process also includes tablet compression, coating, or preparation of injectable solutions.
4. Quality Control and Testing
After formulation, the product undergoes rigorous testing to ensure it meets all quality standards. Quality control measures include:
- Purity Testing: Ensuring that the final product contains the correct amount of active ingredient and is free from contaminants.
- Stability Testing: Ensuring that the product maintains its potency, stability, and efficacy over time.
- Bioavailability Testing: Ensuring that the active ingredient is absorbed properly by the body when consumed or injected.
- Packaging Compliance: Ensuring that the product is safely packaged and labeled according to regulatory guidelines.
5. Packaging
The methotrexate is then packaged in the required forms (e.g., blister packs for tablets, vials for injectables) under strict hygiene and safety conditions. The packaging process ensures that the product is protected from contamination and remains stable during transportation and storage.
6. Distribution
The final packaged product is ready for distribution to hospitals, clinics, pharmaceutical distributors, and retail pharmacies. Efficient logistics and distribution networks are essential to ensure that the product reaches healthcare providers in a timely and cost-effective manner.
Location and Infrastructure
The location of the Methotrexate Manufacturing Plant is crucial for both operational efficiency and regulatory compliance. Ideally, the plant should be situated in an industrial zone with easy access to transportation networks, such as highways, railways, and ports, for the import of raw materials and export of finished products.
Key infrastructure requirements include:
- Manufacturing Area: Space for reactors, mixers, tablet presses, and filling machines.
- Quality Control Laboratory: A dedicated space for performing raw material testing, in-process checks, and final product quality assurance.
- Packaging Area: Space for the packaging of finished products in sterile conditions.
- Storage Facilities: For storing raw materials, in-process products, and finished goods under appropriate conditions.
Regulatory and Safety Compliance
Methotrexate production must comply with several regulations, including:
- Pharmaceutical Regulations: The plant must adhere to pharmaceutical manufacturing laws, including GMP (Good Manufacturing Practices) and FDA guidelines for drug production.
- Environmental Standards: Compliance with environmental laws regarding waste disposal, water usage, and emissions from the manufacturing process.
- Health and Safety: Ensuring the health and safety of employees through appropriate training and protective measures.
FAQs
What is methotrexate used for?
Methotrexate is primarily used in the treatment of cancer (such as leukemia, lymphoma, and breast cancer) and autoimmune diseases (such as rheumatoid arthritis and psoriasis).
How is methotrexate produced?
Methotrexate is synthesized through a series of chemical reactions, followed by formulation into tablets, oral solutions, or injectables, and subjected to rigorous quality control tests.
Is methotrexate available in generic form?
Yes, methotrexate is available as a generic drug and is widely produced by various pharmaceutical companies around the world.
Are there any side effects of methotrexate?
Like any medication, methotrexate can cause side effects, including nausea, fatigue, liver issues, and immunosuppression. It should be used under medical supervision.
What are the regulations for methotrexate manufacturing?
Methotrexate production must comply with stringent regulations, including GMP guidelines, FDA approvals, and environmental standards. Regular inspections and quality testing are required to maintain compliance.
Related Reports
https://www.expertmarketresearch.com.au/reports/australia-automotive-camera-market
https://www.expertmarketresearch.com.au/reports/australia-automotive-pneumatic-actuators-market
https://www.expertmarketresearch.com.au/reports/australia-sugar-market
Media Contact:
Company Name: Claight Corporation
Contact Person: Lewis Fernandas, Corporate Sales Specialist — U.S.A.
Email: sales@expertmarketresearch.com
Toll Free Number: +1–415–325–5166 | +44–702–402–5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au